- Home
- Treatments (Medications)
- Asthma
- Salamol Inhaler
Salamol Inhaler
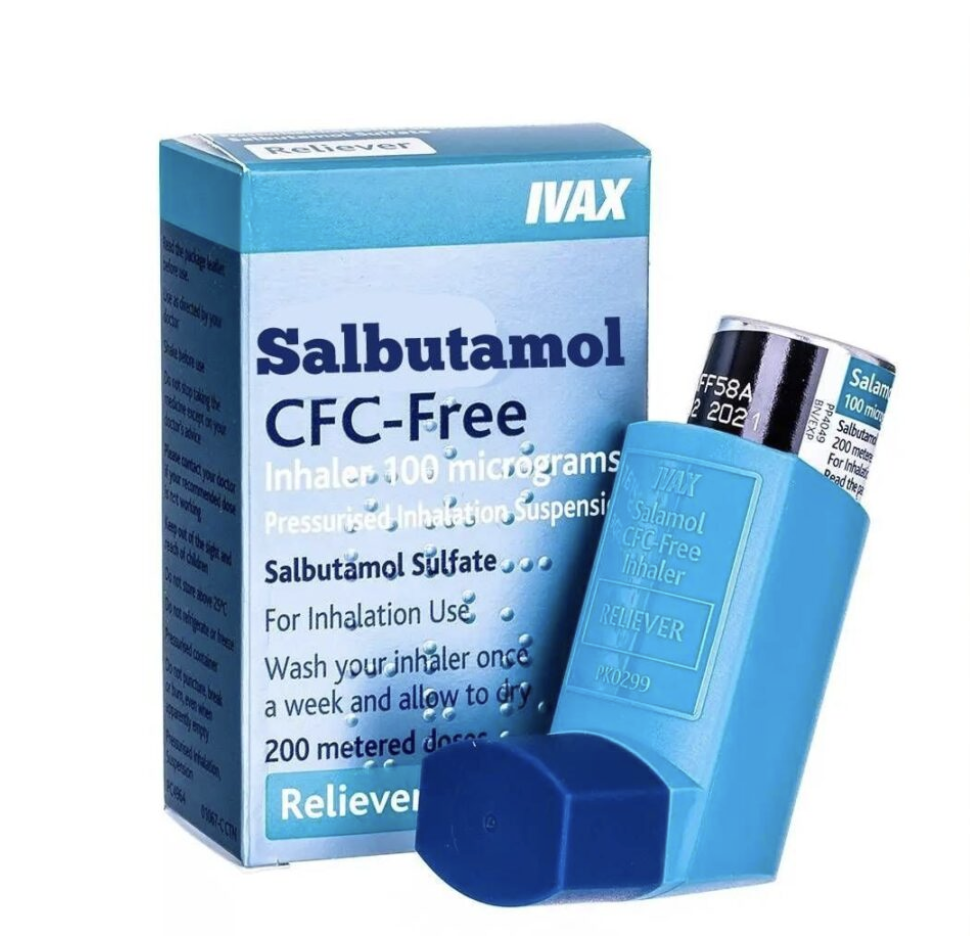

Salamol CFC-Free Inhaler contains salbutamol sulphate, a fast-acting bronchodilator that provides quick relief from asthma symptoms by opening up the airways in the lungs. This reliever inhaler works within minutes and its effects last for 4-6 hours. It is used to treat asthma and chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. Salamol can be used both to prevent asthma symptoms before they occur and to relieve symptoms once they start.
You must complete a short online consultation so that our prescriber can ensure that the treatment is suitable for you.
| Product Name: | Salamol CFC-Free Inhaler |
|---|---|
| Manufacturer: | IVAX Pharmaceuticals Ireland |
| Active Ingredient: | Salbutamol Sulphate |
| Administration: | Oral Inhalation |
| Presentation: | Pressurised Inhalation Suspension |
| Available Strengths: | 100 micrograms per puff |
| Prescription Required: | Yes |
| Dosage: | As needed, up to 4 times daily |
| Suitable for pregnancy? | Caution |
| Use with alcohol: | Yes |
| Strength | Pack Size | Current Price | |
|---|---|---|---|
| 100 micrograms/puff | 200 doses | £12.59 | |
| Important: This is a prescription-only medicine. You must complete an online consultation with one of our UK-registered clinicians to determine if this treatment is appropriate for you. | |||
Swipe left or right to view more information
Table of Contents
- What is Salamol?
- What is Salamol Used For?
- Before Using Salamol
- Salamol Contraindications
- Special Population Considerations
- Salamol and Other Medicines
- Pregnancy & Breastfeeding
- Driving & Machine Operation
- Salamol Ingredients
- How to Use Salamol Inhaler
- Salamol Dosage Guidelines
- Administration Instructions
- Missed Dose Management
- Overdose Information
- Salamol Side Effects
- How to Store Salamol
- Salamol Pack Information
- Salamol FAQs
What is Salamol?
Salamol CFC-Free Inhaler is a pressurised inhalation suspension containing the active ingredient salbutamol sulphate. Salbutamol belongs to a class of medicines called bronchodilators, specifically short-acting beta-2 agonists (SABAs).
Mechanism of Action
Salbutamol works by relaxing the smooth muscles in the airways of the lungs. It selectively targets beta-2 receptors in the bronchial muscles, causing them to relax and allowing the airways to widen (bronchodilation). This makes it easier to breathe by reducing resistance in the respiratory airways and increasing airflow to the lungs.
Patient-Friendly Analogy: Think of your airways as straws that can sometimes become squeezed or narrowed during an asthma attack. Salamol works like gently releasing that squeeze, allowing air to flow freely again.
The medication begins working within minutes of inhalation, with peak effects occurring within 30-60 minutes and lasting for 4-6 hours. Salamol is considered a "reliever" medication because it provides rapid symptom relief rather than preventing asthma attacks.
What is Salamol Used For?
Salamol CFC-Free Inhaler is indicated for the treatment and prevention of bronchospasm in conditions where reversible airway obstruction is a feature.
Approved Indications
- Asthma: For relief of acute bronchospasm and prevention of exercise-induced asthma
- Chronic Obstructive Pulmonary Disease (COPD): Including chronic bronchitis and emphysema
Target Patient Populations
- Adults and adolescents (12 years and older)
- Children under 12 years (with appropriate supervision)
- Patients with reversible airway obstruction
Emergency Warning
If your breathing problems worsen rapidly after using Salamol, or if you experience wheezing following a dose, stop using the inhaler immediately and seek urgent medical attention. This could indicate a paradoxical bronchospasm or serious allergic reaction requiring emergency treatment.
Before Using Salamol
Before starting treatment with Salamol, discuss your medical history with your doctor, particularly if you have any of the following conditions:
Medical History Assessment Checklist
- Heart disease or history of heart problems
- High blood pressure (hypertension)
- Irregular heart rhythm (cardiac arrhythmias)
- Overactive thyroid (thyrotoxicosis)
- Diabetes
- Low potassium levels (hypokalaemia)
- Allergies to salbutamol or any other medicines
Required Baseline Evaluations
Your doctor may recommend the following before prescribing Salamol:
- Lung function tests (spirometry)
- Cardiac evaluation if heart conditions are suspected
- Blood tests to check potassium levels if at risk
- Assessment of asthma control and current medication
Salamol Contraindications
Absolute Contraindications
Do NOT use Salamol if you:
- Are allergic to salbutamol or any other ingredients in this medicine
- Are using it for management of premature labour or threatened miscarriage
Risk Stratification Table
| Condition | Risk Level | Precautions |
|---|---|---|
| Severe heart disease | High | Use with extreme caution; monitor cardiac function |
| Thyrotoxicosis | High | Increased risk of adverse cardiac effects |
| Diabetes | Moderate | Monitor blood glucose; salbutamol may increase levels |
| Mild hypertension | Low-Moderate | Regular blood pressure monitoring recommended |
Special Population Considerations
Elderly Patients
Elderly patients may be more susceptible to the effects of salbutamol, particularly increased heart rate and tremor. Dose adjustment may be necessary based on renal and hepatic function.
Pediatric and Adolescent Patients
Salamol is suitable for children, but an adult should always supervise administration. Children may need help using the inhaler properly. Parents can assist by spraying the aerosol when the child begins to breathe in.
Renal or Hepatic Impairment
No specific dose adjustments are recommended for patients with renal or hepatic impairment, but caution is advised and patients should be monitored for potential side effects.
Salamol and Other Medicines
Tell your doctor or pharmacist about all medicines you are taking, including prescription, over-the-counter, and herbal products.
High-Risk Medication Combinations
Do NOT use Salamol with:
- Propranolol or other non-selective beta-blockers (may counteract the effects of salbutamol)
Medicines That May Interact with Salamol
- Other bronchodilators: Xanthine derivatives (aminophylline, theophylline) - increased risk of side effects
- Diuretics: May worsen hypokalaemia (low potassium)
- Corticosteroids: May enhance hypokalaemic effects
- Digoxin: Risk of digoxin toxicity if hypokalaemia occurs
- Monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants: May potentiate cardiovascular effects
Timing Recommendations
If you are using both a preventer and reliever inhaler, use the preventer inhaler first, wait 5 minutes, then use Salamol if needed for immediate symptom relief.
Pregnancy & Breastfeeding
Pregnancy
Use during pregnancy is NOT recommended unless the expected benefit to the mother outweighs any possible risk to the baby. Some forms of salbutamol can be used for obstetric purposes, but the inhaled preparation MUST NOT be used for management of premature labour or threatened miscarriage.
Breastfeeding
Use during breastfeeding is NOT recommended unless the expected benefit to the mother outweighs any possible risk to the baby. Salbutamol may pass into breast milk and could potentially affect the infant.
Fertility
There is no evidence that salbutamol affects human fertility.
Driving & Machine Operation
Salamol Inhaler is not expected to affect your ability to drive or operate machinery. However, if you experience dizziness, tremor, or other side effects that could impair your abilities, you should avoid these activities until you know how the medication affects you.
Warning Signs to Watch For
- Tremor or shakiness in hands
- Dizziness or lightheadedness
- Palpitations or irregular heartbeat
- Headache
Salamol Ingredients
Active Ingredient
- Salbutamol sulphate (equivalent to 100 micrograms salbutamol per metered dose)
Inactive Ingredients (Excipients)
- Ethanol anhydrous (alcohol) - less than 100mg per puff
- Norflurane (HFA-134a) - propellant
Allergen Warnings
This product contains ethanol (alcohol). If you have a known allergy or sensitivity to alcohol, discuss with your doctor before using this inhaler.
How to Use Salamol Inhaler
Proper technique is essential for Salamol to work effectively. Follow these steps carefully.
Preparation
- Remove the cap from the inhaler
- Check that the mouthpiece is clean
- Shake the inhaler well before each use
Testing Your Inhaler
Before first use or if not used for 5 days or more, test spray the inhaler by firing two puffs into the air.
Salamol Dosage Guidelines
Adults and Adolescents (12 years and over)
| Purpose | Dose | Frequency |
|---|---|---|
| Relief of asthma symptoms | 1-2 puffs (100-200 micrograms) | As needed |
| Prevention of exercise-induced asthma | 2 puffs (200 micrograms) | 10-15 minutes before exercise |
| Long-term treatment | Up to 2 puffs (200 micrograms) | 4 times daily |
Children (under 12 years)
| Purpose | Dose | Frequency |
|---|---|---|
| Relief of asthma symptoms | 1 puff (100 micrograms) | As needed (may increase to 2 puffs if required) |
| Prevention of exercise-induced asthma | 1 puff (100 micrograms) | 10-15 minutes before exercise |
| Long-term treatment | Up to 2 puffs (200 micrograms) | 4 times daily |
Maximum Dose: Do not exceed 8 puffs in 24 hours for any patient. If you need more than 8 puffs per day, consult your doctor as your asthma may not be well controlled.
Administration Instructions
Step-by-Step Guide
- Remove the cap from the inhaler and check the mouthpiece is clean
- Hold the inhaler upright with your thumb on the base and index finger on the top of the canister
- Shake the inhaler vigorously up and down
- Breathe out normally as far as comfortable
- Place the mouthpiece between your lips and form a tight seal
- Start breathing in slowly and deeply, then press down firmly on the canister to release a puff
- Continue to breathe in slowly and deeply
- Remove the inhaler from your mouth and hold your breath for 10 seconds (or as long as comfortable)
- Breathe out slowly
- If another puff is needed, wait about one minute and repeat from step 2
Cleaning Instructions
Clean your inhaler once a week to prevent blockage:
- Remove the mouthpiece cap
- Remove the metal canister from the plastic mouthpiece (do not wash the canister)
- Rinse the plastic mouthpiece and cap under warm running water for at least 30 seconds
- Shake off excess water and allow to dry thoroughly (preferably overnight)
- Reassemble the inhaler once completely dry
Missed Dose Management
Since Salamol is used as needed for symptom relief, there is no fixed dosing schedule. If you forget to use your inhaler before exercise or when symptoms begin, use it as soon as you remember, then continue with your regular routine.
Do NOT take a double dose to make up for a forgotten dose.
Overdose Information
If you accidentally use more Salamol than prescribed, or if someone else uses it without prescription, contact your nearest hospital emergency department or tell your doctor immediately.
Symptoms of Overdose
- Muscle tremor
- Headache
- Rapid heartbeat (tachycardia)
- Palpitations
- Flushing
- Low potassium levels (hypokalaemia)
Management
Take the inhaler and this leaflet with you to the hospital so healthcare professionals know what has been taken. Treatment is supportive and may include monitoring of heart function and potassium levels.
Possible Side Effects
Like all medicines, Salamol can cause side effects, although not everyone gets them.
Serious Side Effects - Seek Immediate Medical Attention
Stop using Salamol and tell your doctor or go to hospital immediately if:
- Your breathing problems worsen or you experience wheezing after a dose
- You have signs of serious allergic reaction (swelling of lips, face, neck; difficulty breathing; skin rash or hives)
- You experience chest pain, shortness of breath, or signs of a heart problem
- You don't feel better after 3 hours or longer following a dose
Common Side Effects (affecting less than 1 in 10 people)
- Tremor (shakiness)
- Headache
- Increased heart rate (tachycardia)
Uncommon Side Effects (affecting less than 1 in 100 people)
- Palpitations
- Mouth and throat irritation
- Muscle cramps
Rare Side Effects (affecting less than 1 in 1,000 people)
- Low potassium levels in blood (hypokalaemia)
- Flushing or redness of face/skin
Very Rare Side Effects (affecting less than 1 in 10,000 people)
- Rapid and irregular heartbeat (atrial fibrillation)
- Other heart rhythm disturbances
- Hyperactivity (especially in children under 12)
Frequency Not Known
- Decreased blood flow to heart (myocardial ischaemia)
Reporting Side Effects: If you get any side effects, talk to your doctor, pharmacist or nurse. You can also report side effects directly via the HPRA at Tel: +353 1 6764971, Website: www.hpba.ie, Email: medsafety@hpba.ie
How to Store Salamol
- Keep out of sight and reach of children
- Do not store above 25°C
- Do not refrigerate or freeze
- Protect from frost, direct sunlight and heat
- Do not expose to temperatures higher than 50°C
- Do not use after the expiry date printed on the carton and canister
- Do not use if the canister is damaged
Disposal
Do not throw away medicines via wastewater or household waste. Return any unused or expired inhalers to your pharmacist for safe disposal.
Pack Information
- Presentation: Pressurised container with metering valve and actuator
- Pack Size: Single inhaler containing 200 metered doses
- CFC-Free: Contains hydrofluoroalkane (HFA-134a) propellant instead of CFCs
- Manufacturer: IVAX Pharmaceuticals Ireland, Waterford, Ireland
- Marketing Authorisation Holder: Imhact Ltd., Dublin, Ireland
Where Can I Buy Salamol Inhaler Online in the UK
Secure Salamol Prescription & Next-Day Delivery Service
Order Salamol with confidence through our UK-registered medical prescribers, who review all requests within 4 working hours. We guarantee same-day prescription approval for eligible patients and dispatch orders placed before 3pm for next-day tracked delivery.
Our Salamol service combines competitive pricing with strict adherence to MHRA safety standards, ensuring your medication is dispensed through GPhC-registered pharmacies. Every purchase includes discreet packaging and a GDPR-compliant consultation process.
Our clinical team ensures:
- Comprehensive medical history review
- Asthma control assessment
- Drug interaction checks
- Personalised dosing guidance
Always consult your GP before starting asthma treatment. Not recommended for children under 4 years without medical supervision.
Salamol (Salbutamol) FAQs
How quickly does Salamol work?
Salamol begins working within minutes of inhalation, with peak effects occurring within 30-60 minutes. The bronchodilator effects typically last for 4-6 hours.
Can I use Salamol if I'm pregnant?
Use during pregnancy is not recommended unless the expected benefit to the mother outweighs any possible risk to the baby. Always consult your doctor before using any medication during pregnancy.
What is the maximum number of puffs I can take in a day?
Do not exceed 8 puffs in 24 hours. If you need more than this, your asthma may not be well controlled and you should consult your doctor for a review of your treatment.
Can children use Salamol Inhaler?
Yes, Salamol can be used by children, but an adult should always supervise administration. Children may need help using the inhaler properly.
How often should I clean my Salamol Inhaler?
You should clean your inhaler at least once a week to prevent blockage. Remove the canister and rinse the plastic mouthpiece and cap under warm running water for 30 seconds, then allow to dry thoroughly before reassembling.
What should I do if my inhaler seems blocked?
If your inhaler seems blocked, clean it according to the instructions. Test spray by firing two puffs into the air after cleaning. If it still doesn't work properly, contact your pharmacist for advice.
Can I use Salamol with a spacer device?
Yes, Salamol is compatible with the Volumatic® Spacer device. Spacers can help improve drug delivery to the lungs, especially for children or those with poor inhaler technique.
What is the difference between Salamol and preventer inhalers?
Salamol is a "reliever" inhaler that provides quick relief from asthma symptoms. Preventer inhalers (usually containing corticosteroids) are used daily to reduce inflammation and prevent asthma attacks from occurring.
Can I use Salamol if I have heart problems?
If you have heart disease, discuss with your doctor before using Salamol. Salbutamol can increase heart rate and may not be suitable for people with certain heart conditions.
Does Salamol contain steroids?
No, Salamol contains salbutamol, which is a bronchodilator, not a steroid. It works by relaxing the muscles around the airways rather than reducing inflammation.
Related Guides
Getting your medication is quick and easy with our simple 4-step process:
Complete Online Consultation
Fill out our secure medical questionnaire about your asthma symptoms and medical history.
Clinical Review
One of our UK-registered clinicians will review your consultation within 24 hours.
Prescription Approval
If appropriate, our clinician will issue a prescription for Salamol Inhaler.
Delivery
Your medication will be dispensed and delivered discreetly to your chosen address.
Consultation Timeline
Most consultations are reviewed within 4-6 hours during business hours. You will receive an email notification once your consultation has been reviewed.
At Chemist Doctor, we respect your privacy and ensure complete discretion at every step.
Discreet Packaging
All medications are delivered in plain packaging with no indication of the contents.
Data Security
Your medical information is protected with bank-level encryption and strict confidentiality protocols.
GP Communication
With your consent, we can inform your NHS GP about your treatment to ensure continuity of care.
GDPR Compliance: We adhere to all UK data protection regulations. Your information will never be shared without your explicit consent, except where required by law.
We offer two reliable delivery options to suit your needs:
| Service | Delivery Time | Cost |
|---|---|---|
| Standard Delivery | 2-3 working days | Free |
| Next-Day Delivery | Next working day if ordered before 2pm | £3.99 |
Delivery FAQ
Do you deliver to all UK addresses?
We deliver to most UK addresses, including Northern Ireland. Some remote locations may have different delivery times.
Can I track my delivery?
Yes, you will receive a tracking number once your order has been dispatched.
What happens if I'm not home?
The courier will leave a card with instructions for redelivery or collection.
Accepted Payment Methods
- Visa, Mastercard, and Maestro debit/credit cards
- Apple Pay and Google Pay
- PayPal
Security & Compliance
All payments are processed through PCI-DSS compliant systems with bank-level encryption. We never store your full payment details on our servers.
Refund Policy for Prescription Medications
Due to regulations surrounding prescription medications, we cannot accept returns or offer refunds once medication has been dispatched, unless there is a quality issue. Unopened medications in their original packaging may be returned for disposal but cannot be resold or reused.
"Fast delivery and the inhaler works exactly as expected. Much cheaper than buying from my local pharmacy."
"Quick consultation process. The inhaler arrived the next day. Only minor side effects of slight hand tremor."
Purchased Salamol from us? Share your experience to help others.